Gout is a type of inflammatory arthritis caused by a condition called hyperuricemia.1 Hyperuricemia happens when there is too much uric acid in the body.1 When hyperuricemia becomes severe, uric acid crystals can build up in the joints, causing pain, swelling and gout flares.1 Approximately 8.3 million Americans are currently living with this painful disease.1
A progressive disease
Because uric acid builds up over time, it may take months or even years for gout symptoms to appear.2 But when the uric acid level ultimately spikes, it can trigger a gout flare.2
For most people, a gout flare lasts three to 10 days.2 Another may not occur for months or even years.2 Eventually, however, flares become more frequent, severe and long-lasting.2 Joint damage may also occur, which can lead to loss of mobility.2
Once gout flares become a chronic problem, help from a doctor may be necessary to prevent future flares.3
How Mitigare® (colchicine) 0.6 mg Capsules can help prevent gout flares in adults
, the active ingredient in Mitigare®, has been used for centuries to prevent gout flares.4 It was originally derived from the autumn crocus (Colchicum autumnale), and there is evidence that it was used more than 2000 years ago in ancient Greece.4 Since then, colchicine has been proven effective in helping prevent flares in adults with gout.5 The safety and effectiveness of Mitigare for acute treatment of gout flares during prophylaxis has not been studied. Mitigare is not an analgesic medication and should not be used to treat pain from other causes.
Gout flare prevention
Adults who took colchicine 0.6 mg daily had fewer gout flares than patients who did not. They also had fewer gout flares as time went on.5
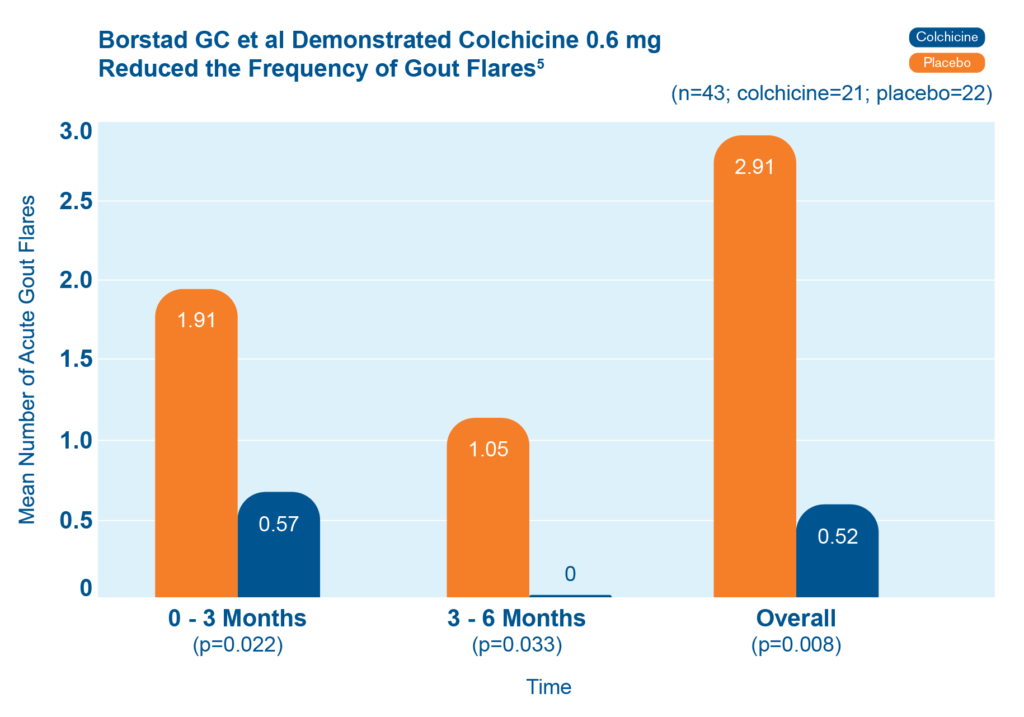
43 patients starting allopurinol were randomized to receive colchicine 0.6 mg or placebo for up to 6 months. The chart shows mean number of acute gout flares at the 0-3 and 3-6 month time periods, and overall (n=43; colchicine=21, placebo=22)
Well tolerated in adults
In a six-month study of adult patients with recurrent gout, colchicine 0.6 mg was well tolerated5:
- The most common adverse reactions were gastrointestinal symptoms, including diarrhea, nausea, vomiting and abdominal pain.
- Colchicine 0.6 mg capsules are contraindicated in patients with renal or hepatic impairment who are currently prescribed drugs that inhibit both P-gp and CYP3A4.
A pop of color
The distinctive two-tone blue capsule may be easier for you to identify, especially if you take other daily medications.6,7 The FDA says that older adults who take more than one medicine should be able to tell them apart by size, shape, color and form (tablet or capsule).7
Don’t wait
Prompt treatment of gout is important. If untreated, the risk of a second gout flare after one year is 62 percent.8 The risk of a second flare increases to 78 percent after two years, and 93 percent after 10 years.8
If gout is untreated for a long period, say 10 years or more, it can become very severe and even disabling.8 By this time, the disease may have permanently damaged the affected joints and even the kidneys.9
Talk with your doctor
If you think you may have gout, talk with your doctor. He or she may be able to recommend lifestyle changes and/or prescribe medicine that can help you prevent gout flares.
Get True Blue Savings
If your doctor prescribes Mitigare® or Generic Colchicine 0.6 mg Capsules, the True Blue Savings program provides the first fill for as little as $0 and offers $5 refills for eligible patients.*
*For all eligible patients 18 years or older who are legal residents of the United States or Puerto Rico. First 30 days are as little as $0 only for eligible patients. Maximum savings of $65 on the first fill and $50 on refills. Please see complete Terms and Conditions for details.
Important Safety Information
Colchicine 0.6 mg capsules are contraindicated in patients with renal or hepatic impairment who are currently prescribed drugs that inhibit both P-gp and CYP3A4. Combining one of these dual inhibitors, or a medication that inhibits either P-gp or CYP3A4, with colchicine has resulted in life-threatening or fatal colchicine toxicity. Patients with both renal and hepatic impairment should not use Mitigare®.
Fatal overdoses have been reported with colchicine in adults and children. Keep Mitigare® out of the reach of children. Blood dyscrasias such as myelosuppression, leukopenia, granulocytopenia, thrombocytopenia and aplastic anemia have been reported with colchicine used in therapeutic doses. Monitor for toxicity and if present consider temporary interruption or discontinuation of colchicine. Please see full Prescribing Information and Medication Guide for Mitigare® for complete product details.
NOTE: This article was not written by a medical professional and is not intended to substitute the guidance of a physician. These are not West-Ward’s recommendations for gout flare prevention, but rather facts and data collected from various reliable medical sources. For a full list of resources and their attributing links, see below.
