People with gout often have other medical conditions2:
- Obesity
- Metabolic syndrome
- Diabetes Mellitus
- Hypertension
- Hyperlipidemia
- Heart failure (CHF)
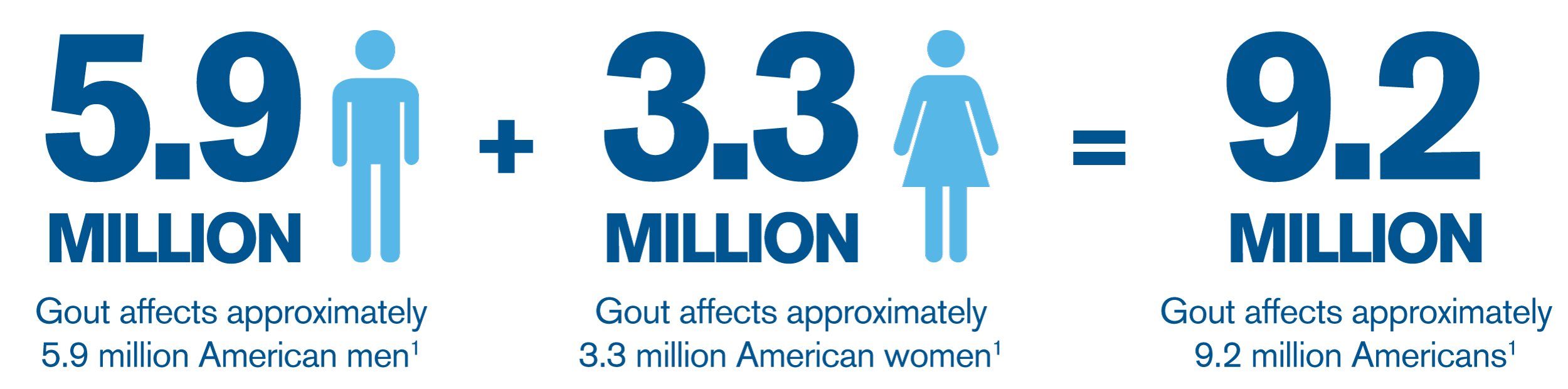
Although the majority of people who suffer with gout are men, many women are affected by the disease.1
People with gout often have other medical conditions2:
Gout may present differently in male and female patients.3 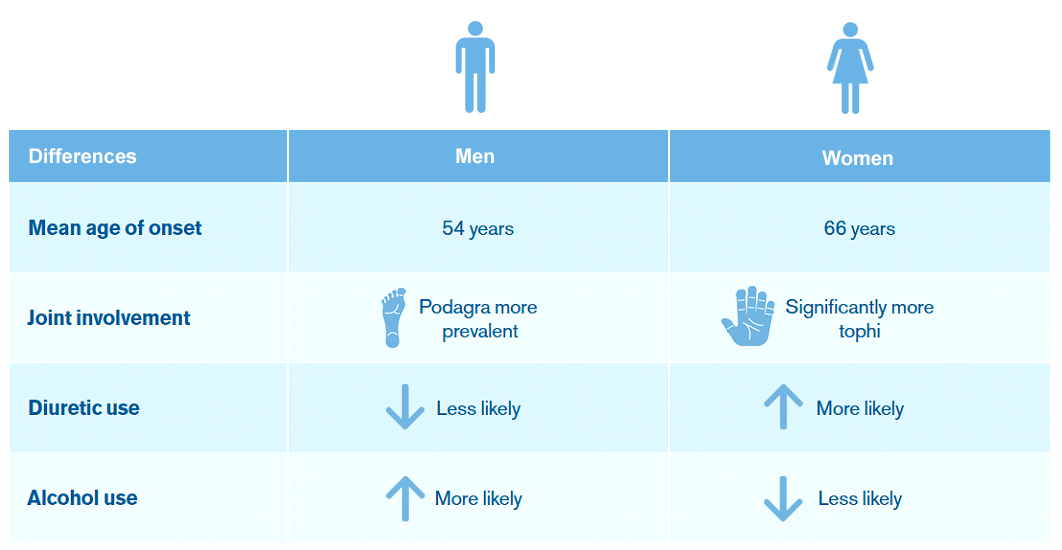
The table above was created by Hikma Pharmaceuticals USA Inc. based on data from a systematic review of the clinical features of men and women with gout. The aim of the review was to evaluate the available studies on the sex differences in clinical features of gout. Nine articles were selected for inclusion in the review.
Mitigare® (Colchicine) 0.6 mg and Generic Colchicine Capsules may be appropriate for patients with4:
Mitigare® may not be right for all adult patients and is contraindicated in patients with renal or hepatic impairment who are currently prescribed drugs that inhibit both P-gp and CYP3A4. Combining these dual inhibitors with colchicine in patients with renal or hepatic impairment has resulted in life-threatening or fatal colchicine toxicity. Patients with both renal and hepatic impairment should not be given Mitigare.® Please see full Prescribing Information and Medication Guide for complete product information.

Your patients may benefit from information on how to prevent their gout flares. Share our Gout Blog with them for a variety of healthy living tips and advice.
GOUT BLOG