Aiming for Fewer Gout Flares
Colchicine is the active ingredient in a medicine called Mitigare®.2 It has been proven effective in helping prevent flares in adults with gout.2
Adults who took colchicine 0.6 mg daily had fewer gout attacks than patients who did not.3 Adults who took colchicine also had fewer gout attacks as time went on.3
Colchicine Prophylaxis Can Reduce the Frequency of Gout Flares3
In one study, 43 patients starting allopurinol were randomized to receive colchicine 0.6 mg or placebo for up to 6 months. Over the study period, the mean number of gout attacks in patients receiving colchicine plus allopurinol was 0.52, compared with 2.91 gout attacks amont patients receiving placebo.3
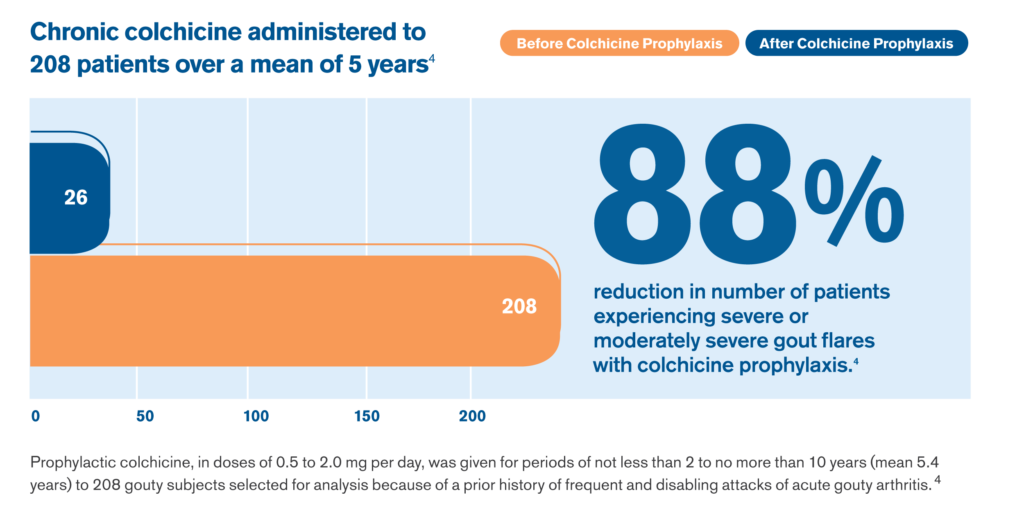
Colchicine Prophylaxis Can Reduce the Severity of Gout Attacks in Most Patients4
Safety Profile
Colchicine is the active ingredient in Mitigare®. In a 6-month study of patients with recurrent gout, colchicine 0.6 mg was well tolerated.3
- The most common adverse reactions were gastrointestinal symptoms, including diarrhea, nausea, vomiting and abdominal pain.2
- Colchicine 0.6 mg capsules are contraindicated in patients with renal or hepatic impairment who are currently prescribed drugs that inhibit both P-gp and CYP3A4.2 Please see Important Safety Information below.
What else can I do to manage my gout attacks?
To stay healthy and minimize the impact of gout attacks on your life, follow your doctor’s advice and take medicines as prescribed. It may also help to5:
- Drink plenty of non-alcoholic and non-sugary beverages, especially water. These kinds of fluids help remove uric acid from the body.
- Avoid foods containing purines. Foods that are high in purines include red meat, organ meats and certain kinds of seafood.
- Maintain a healthy body weight through diet and exercise.
Discover more helpful information in our Lifestyle Tips and Gout Flare Prevention Blog.

