Anyone who has ever had a gout flare knows how excruciating the condition can be.1 However, the agony of gout isn’t necessarily the most significant concern associated with the disease.2 In addition to painful attacks, the risks of uncontrolled gout can include joint disfigurement and destruction, kidney problems, hypertension and heart disease. Uncontrolled gout can even negatively affect quality of life.2 Read on to learn why it is so important to keep gout under control and find out how.
Joint disfigurement and destruction
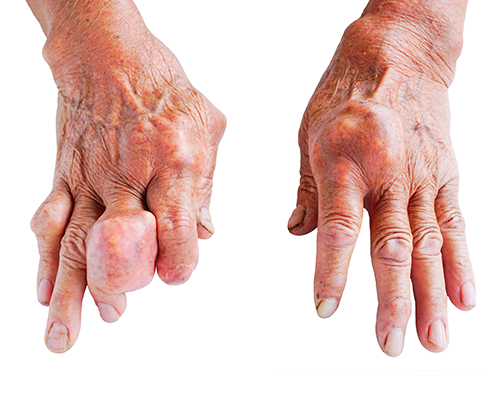
Gout is caused by excess uric acid in the bloodstream.1 If uric acid levels stay high for a long time, urate crystals can build up in the joints.2 In some patients, these crystals grow into large, whitish nodules called tophi.1,3 Often visible under the skin, these unsightly lumps can appear around the joints as well as in the surrounding tissue.3 Over time, tophi can affect your ability to function normally, especially if they develop in the hands and feet.4 Once gout disease becomes chronic, the joints may be permanently damaged and deformed.3
Kidney stones and kidney disease
Urate crystal buildup in the kidneys can lead to all kinds of problems, including kidney stones.5 In addition to causing unbearable pain, kidney stones can be dangerous because they can block the urinary tract and lead to infection.5 If left untreated, kidney stones can even result in chronic kidney disease and ultimately kidney failure.5
Hypertension and heart disease
Many studies have shown that there is a link between uric acid and heart disease.6 People who have gout are more likely to have heart health issues, including blocked arteries, heart attack, stroke and heart failure.6 According to the Gout Education Society, research has shown that having untreated gout can double a person’s risk for heart attack or stroke.6
Poor quality of life
Gout can take a tremendous toll on the lives of those who suffer with it.7 A 2019 survey conducted by the Gout Education Society revealed that four in 10 people say their quality of life is not as good because of gout, and one in four says they feel that gout controls their life.7 These data support the organization’s 2016 survey findings, which revealed that nine out of 10 people with gout worry about it, and more than four in 10 say they can’t imagine anything more painful than a gout attack.8
How you can protect against uncontrolled gout
Although gout is considered a serious disease, only about a third of patients get the gout treatment they need to avoid future flares and complications.7 If you have gout but are not visiting your doctor regularly, schedule checkups every six months and have your uric acid levels checked at each appointment.9 A healthy uric acid level is 6.0 mg/dL, so your doctor may recommend a urate-lowering therapy (ULT) such as allopurinol if you are not already taking one.10 He or she may also recommend that you begin colchicine therapy with a medication called Mitigare® (Colchicine) 0.6 mg Capsules (or Generic Colchicine Capsules) to help prevent gout flares.11,12
Mitigare® is a registered trademark of Hikma Pharmaceuticals USA Inc.
Colchicine 0.6 mg capsules are contraindicated in patients with renal or hepatic impairment who are currently prescribed drugs that inhibit both P-gp and CYP3A4. Combining these dual inhibitors with colchicine in patients with renal or hepatic impairment has resulted in life-threatening or fatal colchicine toxicity. Patients with both renal and hepatic impairment should not be given Mitigare®.
Fatal overdoses have been reported with colchicine in adults and children. Keep Mitigare® out of the reach of children.
Blood dyscrasias such as myelosuppression, leukopenia, granulocytopenia, thrombocytopenia and aplastic anemia have been reported with colchicine used in therapeutic doses.
Monitor for toxicity and, if present, consider temporary interruption or discontinuation of colchicine.
Drug interaction with dual P-gp and CYP3A4 inhibitors: Co-administration of colchicine with dual P-gp and CYP3A4 inhibitors has resulted in life-threatening interactions and death.
Neuromuscular toxicity and rhabdomyolysis may occur with chronic treatment with colchicine in therapeutic doses, especially in combination with other drugs known to cause this effect. Patients with impaired renal function and elderly patients (including those with normal renal and hepatic function) are at increased risk. Consider temporary interruption or discontinuation of Mitigare®.
The most commonly reported adverse reactions with colchicine are gastrointestinal symptoms, including diarrhea, nausea, vomiting and abdominal pain.
Please see the full Prescribing Information and Medication Guide for Mitigare® for complete product details.
NOTE: This article was not written by a medical professional and is not intended to substitute for the guidance of a physician. These are not Hikma’s recommendations for gout flare prevention, but rather facts and data collected from various reliable medical sources. For a full list of resources and their attributing links, see below.
