Approximately 8.3 million Americans are currently living with gout.1 Certain lifestyle changes may help manage this painful disease, but medication is the most proven, effective preventive therapy against gout flares.2
One such medication your doctor may prescribe is colchicine. , derived from a plant known as the autumn crocus or meadow saffron, was discovered centuries ago.3 Colchicine is the active ingredient in Mitigare® (colchicine) 0.6 mg Capsules. The safety and effectiveness of Mitigare® for acute treatment of gout flares during prophylaxis has not been studied. Mitigare® is not an analgesic medication and should not be used to treat pain from other causes.
Preventive therapy with colchicine
In a clinical study, patients who took colchicine 0.6 mg daily had fewer gout flares than patients who did not.4 Patients who took colchicine also had fewer gout flares as time went on.4
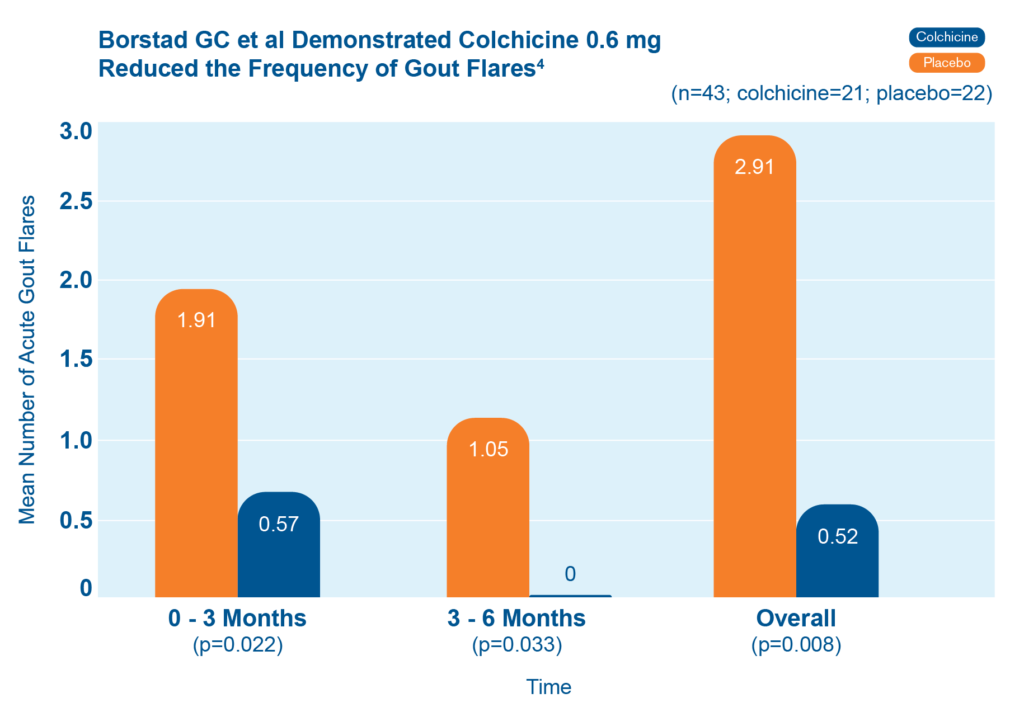
Forty-three patients starting allopurinol were randomized to receive colchicine 0.6 mg or placebo for up to 6 months.4 The chart shows the mean number of acute gout flares at the 0-3 and 3-6 month time periods, and overall (n=43; colchicine=21, placebo=22).4
The same study showed that colchicine 0.6 mg was well tolerated.4 The most commonly reported adverse reactions associated with colchicine are gastrointestinal symptoms, including diarrhea, nausea, vomiting and abdominal pain.5
Stay on preventive therapy for as long as your doctor recommends
In addition to helping you avoid the agonizing pain associated with gout flares, there are many reasons why it is important to remain on preventive therapy.
Increasingly frequent flares
After uric acid levels have been high for many years, flares which were once infrequent begin to occur regularly. 6 About 60 percent of people who have had a flare will have another within the next 12 months.6 More than 80 percent will have another flare within the next three years.6 Meanwhile, the pain associated with these increasingly frequent flares can last longer and become even more severe.6
Risk of permanent damage
If gout is untreated for a long period, say 10 years or more, it can become very severe and even disabling.7 In some cases, the disease can permanently damage the affected joints and even the kidneys.8
Help reaching uric acid goals
Urate-lowering therapy (ULT) with prescription medications such as allopurinol, Uloric® (febuxostat), Zurampic® (lesinurad) or Krystexxa® (pegloticase) can be an important part of gout management.9 However, some patients who take these medicines experience more frequent gout flares.9 These flares may be caused by the uric acid crystals in the joints dissolving as the uric acid level in the bloodstream goes down.9
Research suggests that adult gout patients should be made aware of the potential increase in flares associated with ULT.9 It also shows that flare prevention with colchicine may help adult patients remain on ULT and ultimately achieve their gout treatment goals.9
Flare prevention is the standard of care
To prevent flares, the American College of Rheumatology (ACR) Guidelines for Management of Gout recommends that patients begin an anti-inflammatory agent before or during ULT.10 The ACR recommends low-dose colchicine (0.5 to 0.6 mg orally once or twice a day) as a first-line option for gout flare prevention.10
Important Safety Information
Colchicine 0.6 mg capsules are contraindicated in patients with renal or hepatic impairment who are currently prescribed drugs that inhibit both P-gp and CYP3A4. Combining one of these dual inhibitors, or a medication that inhibits either P-gp or CYP3A4, with colchicine has resulted in life-threatening or fatal colchicine toxicity.
Patients with both renal and hepatic impairment should not use Mitigare®.
Fatal overdoses have been reported with colchicine in adults and children. Keep Mitigare® out of the reach of children.
Blood dyscrasias such as myelosuppression, leukopenia, granulocytopenia, thrombocytopenia and aplastic anemia have been reported with colchicine use.
Monitor for toxicity and if present consider temporary interruption or discontinuation of colchicine.
Please see full Prescribing Information and Medication Guide for Mitigare® for complete product details.
Find out more about preventive therapy
To find out more about preventing gout flares with Mitigare, ask your doctor or visit www.Mitigare.com.
NOTE: This article was not written by a medical professional and is not intended to substitute the guidance of a physician. These are not West-Ward’s recommendations for gout flare prevention, but rather facts and data collected from various reliable medical sources. For a full list of resources and their attributing links, see below.
All registered trademarks are the property of their respective owners.
