- Colchicine 0.6 mg capsules are contraindicated in patients with renal or hepatic impairment who are currently prescribed drugs that inhibit both P-gp and CYP3A4. Combining these dual inhibitors with colchicine in patients with renal or hepatic impairment has resulted in life-threatening or fatal colchicine toxicity. Patients with both renal and hepatic impairment should not be given Mitigare®.
- Fatal overdoses have been reported with colchicine in adults and children. Keep Mitigare® out of the reach of children.
- Blood dyscrasias such as myelosuppression, leukopenia, granulocytopenia, thrombocytopenia, and aplastic anemia have been reported with colchicine used in therapeutic doses.
- Monitor for toxicity and if present consider temporary interruption or discontinuation of colchicine.
- Drug interaction with dual P-gp and CYP3A4 inhibitors: Co-administration of colchicine with dual P-gp and CYP3A4 inhibitors has resulted in life-threatening interactions and death.
- Neuromuscular toxicity and rhabdomyolysis may occur with chronic treatment with colchicine in therapeutic doses, especially in combination with other drugs known to cause this effect. Patients with impaired renal function and elderly patients (including those with normal renal and hepatic function) are at increased risk. Consider temporary interruption or discontinuation of Mitigare®.
- The most commonly reported adverse reactions with colchicine are gastrointestinal symptoms, including diarrhea, nausea, vomiting, and abdominal pain.
Indication
Mitigare® is indicated for prophylaxis of gout flares in adults. The safety and effectiveness of Mitigare for acute treatment of gout flares during prophylaxis has not been studied.
Mitigare® is not an analgesic medication and should not be used to treat pain from other causes.
For Full Prescribing Information please CLICK HERE and for Medication Guide CLICK HERE.
You are encouraged to report negative side effects of prescription drugs to the FDA.
Visit www.fda.gov/medwatch, or call 1-800-FDA-1088.
Manufactured by: West-Ward Columbus Inc., Columbus, OH 43228

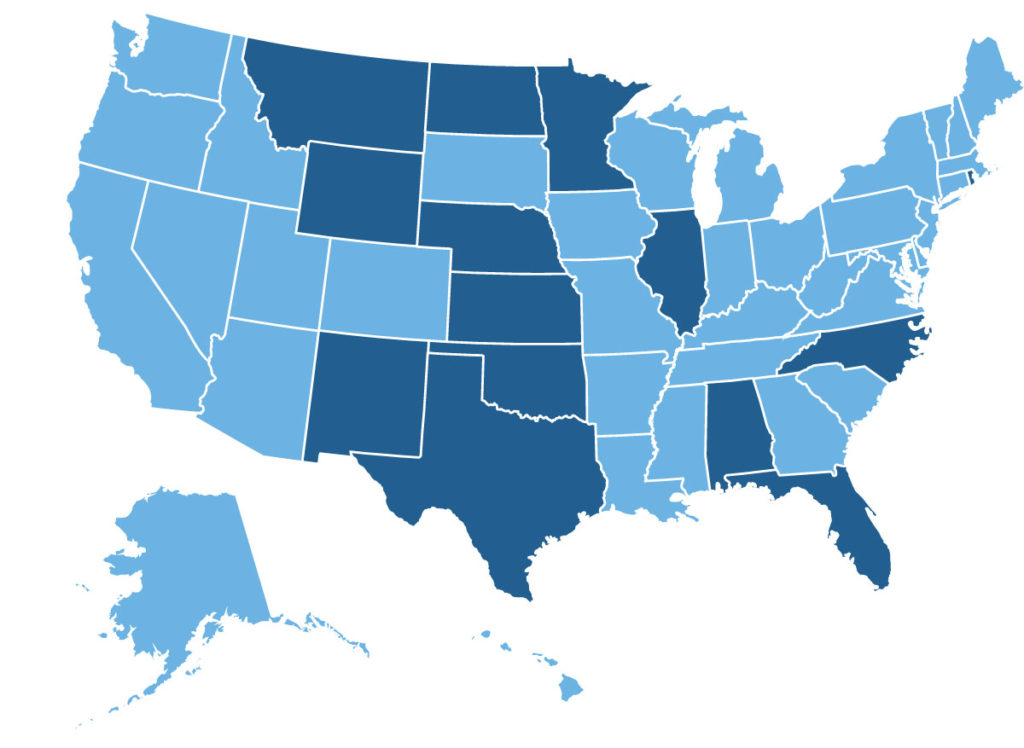 Hikma announced the addition of 14 BCBS Plans that have made Mitigare® (Colchicine) 0.6 mg Capsules preferred on their commercial formularies.
Hikma announced the addition of 14 BCBS Plans that have made Mitigare® (Colchicine) 0.6 mg Capsules preferred on their commercial formularies.