Have you ever suffered a gout flare? If you have, it is certainly understandable if the threat of a sudden and intensely painful gout attack worries you more than anything else about the condition. But have you ever considered how gout could affect your bones and joints? Read on to learn more about the disease and some steps you can take toward maintaining bone and joint health with gout.
The underlying cause of gout
Gout is a condition caused by excess uric acid in the bloodstream.1 When the uric acid level in the blood remains high for a long time, tiny needle-shaped urate crystals can build up around a joint.1 Occasionally, these crystals can trigger a sudden and intensely painful episode known as a gout flare.2 But a painful gout flare isn’t the only trouble these crystals can cause. If gout is not managed effectively, these tiny crystals can slowly and silently grow into a bigger problem—large, chalky lumps under the skin called tophi.2
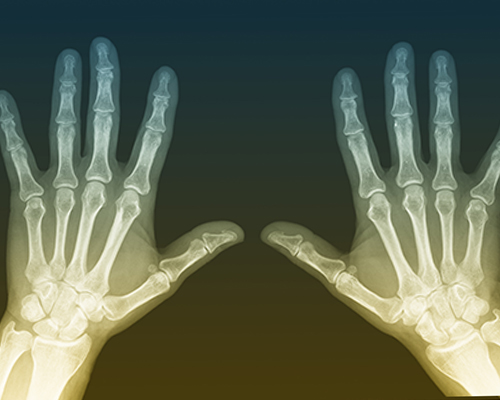
The potential impact of tophi on bones and joints
Because tophi start out so small, they sometimes go undetected for months—even years.3 If the underlying cause of gout (ie, a high uric acid level) goes untreated, these tiny urate crystals can grow into hard lumps in the affected joint and surrounding tissues.2 In fact, these lumps can grow so large, they can affect the joint’s ability to move and function normally (this is especially true of tophi that develop in the hands or feet).4 In some severe cases, tophi can become so big, they break through the skin and can become infected.5 Unless the uric acid level is reduced, gout can become chronic and the joints can become permanently damaged and deformed.5 Taking control of the disease by reducing its destructive potential is important for maintaining bone and joint health with gout.5
Visit your doctor
Only about one in three people with gout actually get the treatment they need to avoid future flares and prevent complications.6 You owe it to yourself to visit your doctor regularly and take steps toward maintaining your bone and joint health with gout. Schedule checkups every six months and have your uric acid level checked at each appointment.7 If your uric acid level is higher than 6.0 mg/dL, your doctor may recommend a urate-lowering therapy (ULT) if you are not already taking one.8 Your doctor may also recommend that you begin a colchicine therapy with a medication called Mitigare® (Colchicine) 0.6mg Capsules or ) to help prevent gout attacks.9
Tips for maintaining bone and joint health with gout
Consider these health tips to help you live better with gout:
- Eat a well balanced diet—Whether you have gout or not, eating a well balanced diet is an important part of a healthy lifestyle.10 The Gout-Friendly Diet Cheat Sheet is a great resource you can quickly review to learn more about the best foods for people with gout.
- Drink water—Consume at least 8 glasses of water per day.11 Doing so can help flush the excess uric acid out of your system.11
- Maintain a healthy weight—Maintaining a healthy weight (or losing weight, if you need to) may help reduce the amount of uric acid in your bloodstream.1 (Do not fast or attempt rapid weight loss, however, as these may temporarily increase your uric acid level and raise your risk of a flare.1)
- Take your medications—According to doctors at the Mayo Clinic, medication is often the most effective way to treat gout and prevent attacks.12
Gout Friendly Diet Cheat Sheet
Discover the benefits of a gout-friendly diet that may reduce your risk of future flares.
INFOGRAPHIC
Mitigare® is a registered trademark of Hikma Pharmaceuticals USA Inc.
Colchicine 0.6mg capsules are contraindicated in patients with renal or hepatic impairment who are currently prescribed drugs that inhibit both P-gp and CYP3A4. Combining these dual inhibitors with colchicine in patients with renal or hepatic impairment has resulted in life-threatening or fatal colchicine toxicity. Patients with both renal and hepatic impairment should not be given Mitigare®.
Fatal overdoses have been reported with colchicine in adults and children. Keep Mitigare® out of the reach of children.
Blood dyscrasias such as myelosuppression, leukopenia, granulocytopenia, thrombocytopenia and aplastic anemia have been reported with colchicine used in therapeutic doses.
Monitor for toxicity and, if present, consider temporary interruption or discontinuation of colchicine.
Drug interaction with dual P-gp and CYP3A4 inhibitors: Co-administration of colchicine with dual P-gp and CYP3A4 inhibitors has resulted in life-threatening interactions and death.
Neuromuscular toxicity and rhabdomyolysis may occur with chronic treatment with colchicine in therapeutic doses, especially in combination with other drugs known to cause this effect. Patients with impaired renal function and elderly patients (including those with normal renal and hepatic function) are at increased risk. Consider temporary interruption or discontinuation of Mitigare®.
The most commonly reported adverse reactions with colchicine are gastrointestinal symptoms, including diarrhea, nausea, vomiting and abdominal pain.
Please see the full Prescribing Information and Medication Guide for Mitigare® for complete product details.
NOTE: This article was not written by a medical professional and is not intended to substitute for the guidance of a physician. These are not Hikma’s recommendations for gout flare prevention, but rather facts and data collected from various reliable medical sources. For a full list of resources and their attributing links, see below.

