As you’re probably aware, prescription drug prices in the US have been creeping up for years.1 Some have been rising more quickly than others, however.1 The price of Colcrys® (colchicine, USP) tablets, for example, has increased by more than 40 percent since 2013.2 Keep reading to find out more about the cost of Colchicine and how it has changed in recent years.
A federal initiative
In 2006, the US Food and Drug Administration (FDA) announced a new program called the Unapproved Drugs Initiative (UDI).3 The UDI required drug manufacturers to get FDA approval for their products by proving their safety and effectiveness through costly research.3 Manufacturers who chose not to comply were forced to remove their products from the market.3 Even though Colchicine products had been prescribed for decades to prevent gout attacks, none of them were FDA-approved prior to 2009.4
A radical price increase in the cost of colchicine
In 2009, the FDA approved Colcrys® (colchicine, USP) 0.6 mg tablets.4 Colcrys entered the market at about $5 per tablet—a significantly higher cost of colchicine than the pre-existing generics, which cost about nine cents per pill.4 But when the FDA ultimately banned the sale of unapproved Colchicine products in 2010, patients were forced to pay about 50 times more than they had before for their gout flare prevention therapy.4
A new alternative
In 2014, Hikma5 received FDA approval for the first and only alternative colchicine product for gout flare prevention in adults—Mitigare® (Colchicine) 0.6 mg Capsules. Mitigare® and launched the following year, generating competition and introducing the possibility for people with gout to save on the cost of colchicine therapy compared to Colcrys®. Mitigare® and Generic Colchicine Capsules remain the lowest-priced Colchicine products available today.2
A unique co-pay program
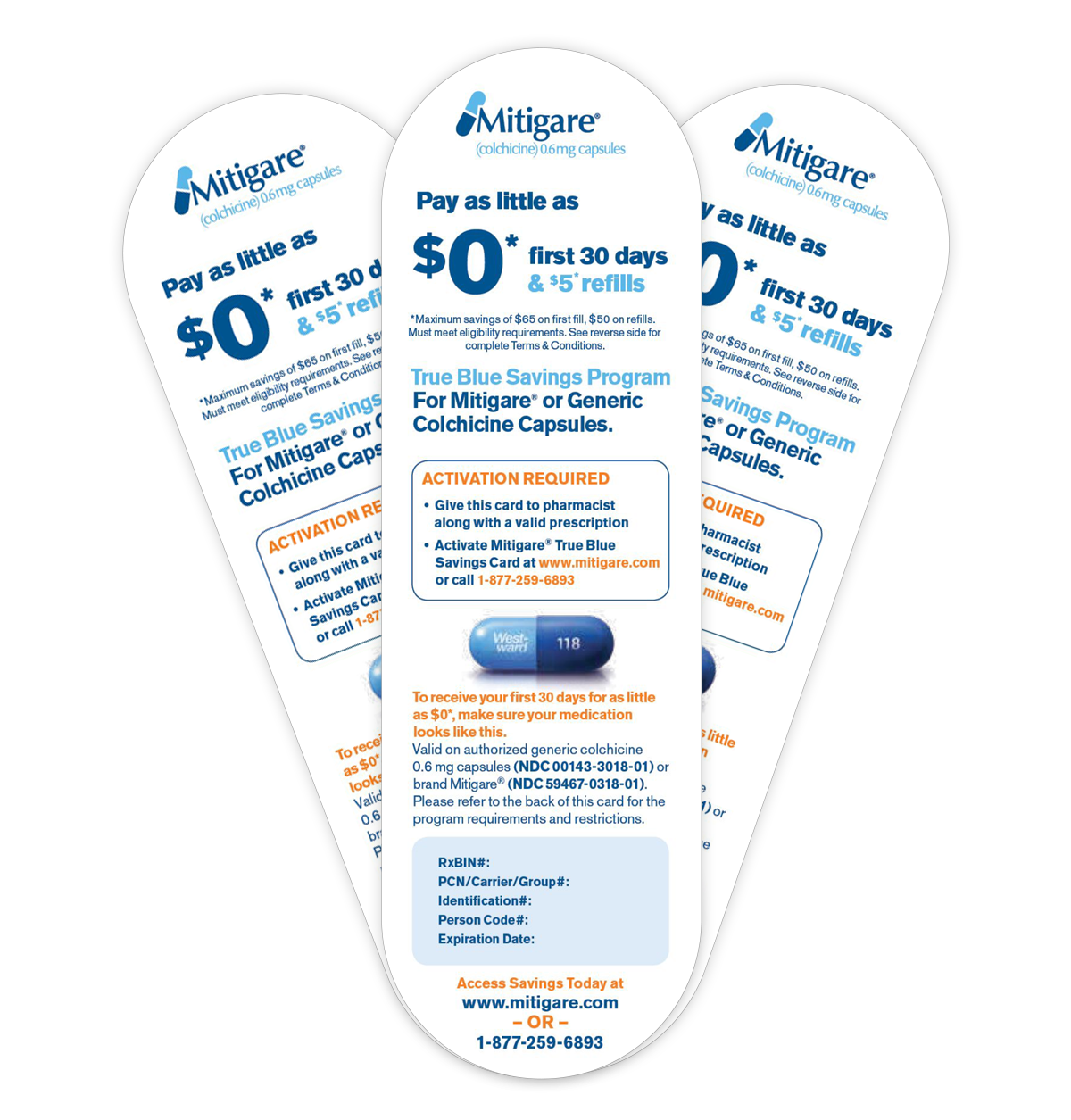
Hikma helps patients save even more on their Colchicine therapy through the True Blue Savings Program. The True Blue Savings Program is the only co-pay program that helps eligible patients save on Mitigare® and Generic Colchicine Capsules.* Upon enrollment, eligible patients can receive their first 30 days of Mitigare® or Generic Colchicine Capsules for as little as $0 and refills for as little as $5 per month.* Find out if you are eligible to save on Colchicine therapy with True Blue Savings now.
A broad spectrum of coverage
Another way Hikma is making Colchicine therapy more affordable is by ensuring broad access to the drug for the people who need it. Mitigare® (Colchicine) 0.6 mg Capsules and Generic Colchicine Capsules are covered by 82 percent of major health plans†, including:
- United Health Group®
- CVS Caremark®
- Cigna® Health Plans, Inc.
- AetnaSM
- Express Scripts® PBM
- CVS® SilverScript®
- Most BCBS® Plans, State Medicaid and Wellcare Part D
An affordable prevention option
By lowering the overall cost of treatment, Hikma is committed to improving access to gout flare prevention. To find out more about Mitigare® and Generic Colchicine Capsules and the True Blue Savings Program, see complete Terms & Conditions and eligibility requirements.
Mitigare® True Blue Savings Card
Eligible patients can receive Mitigare® or Generic Colchicine Capsules for as little as $0 for the first 30 days and get refills as low as $5.*
DOWNLOAD
*For all eligible patients 18 years or older who are legal residents of the United States or Puerto Rico. First 30 days are as little as $0 only for eligible patients. Maximum savings of $65 on the first fill and $50 on refills. Please see complete Terms and Conditions.
†Managed Markets Insight & Technology, LLC database and formulary status as of January 2020.
Mitigare® is a registered trademark of Hikma Pharmaceuticals USA Inc.
Colcrys® is a trademark of Takeda Pharmaceuticals U.S.A., Inc., registered with the US Patent and Trademark Office and used under license by Takeda Pharmaceuticals America, Inc.
Important Safety Information
Colchicine 0.6 mg capsules are contraindicated in patients with renal or hepatic impairment who are currently prescribed drugs that inhibit both P-gp and CYP3A4. Combining these dual inhibitors with colchicine in patients with renal or hepatic impairment has resulted in life-threatening or fatal colchicine toxicity. Patients with both renal and hepatic impairment should not be given Mitigare®.
Fatal overdoses have been reported with colchicine in adults and children. Keep Mitigare® out of the reach of children.
Blood dyscrasias such as myelosuppression, leukopenia, granulocytopenia, thrombocytopenia, and aplastic anemia have been reported with colchicine used in therapeutic doses.
Monitor for toxicity and if present consider temporary interruption or discontinuation of colchicine.
Drug interaction with dual P-gp and CYP3A4 inhibitors: Co-administration of colchicine with dual P-gp and CYP3A4 inhibitors has resulted in life-threatening interactions and death.
Neuromuscular toxicity and rhabdomyolysis may occur with chronic treatment with colchicine in therapeutic doses, especially in combination with other drugs known to cause this effect. Patients with impaired renal function and elderly patients (including those with normal renal and hepatic function) are at increased risk. Consider temporary interruption or discontinuation of Mitigare®.
Please see the full Prescribing Information and Medication Guide for Mitigare® for complete product details.
NOTE: This article was not written by a medical professional and is not intended to substitute for the guidance of a physician. These are not Hikma’s recommendations for gout flare prevention, but rather facts and data collected from various reliable medical sources. For a full list of resources and their attributing links, see below.