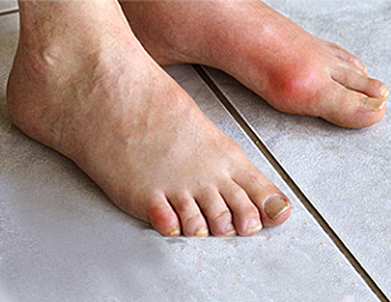
The big toe often is where gout attacks first. Gout attacks also can occur in the heels, instep, ankles, knees, elbows, hips, thumbs, and fingers. Symptoms of gout include intense pain that often begins in the middle of the night, tenderness, redness and/or warmth in the affected joints, and lingering discomfort.1 If you’ve experienced what might be a gout attack or gout flare, see your doctor to learn more about gout and the prescription medication available to prevent gout flares. Here is some important information that you need to know if your doctor has diagnosed you with gout.
Gout is a type of inflammatory arthritis which results from too much uric acid building up in the body, leading to the chronic swelling of joints. As a result, adults with gout may have a limited range of motion. This disease affects 8.3 million Americans.2
Uric acid builds up over time, so it may take months or even years for symptoms to first appear. Once the initial attack has occurred, however, flares usually return, lasting from three to 10 days. While the second flare may not occur again for months or even years, eventually flare-ups become more frequent and even more painful. Ask your doctor about preventing gout flares.2
Gout flares
There are several stages of gout. The ultimate and most serious stage involves gout flares, which may develop in people whose uric acid levels have been high for years. Once infrequent, flares now occur regularly, with pain that’s more severe and long-lasting. Without treatment, it is possible that joint damage, as well as a loss of mobility, will occur. Gout flares are also associated with other serious health issues, including cardiovascular disease and diabetes.3
Once uric acid levels in the blood reach too high a level, gout has become a chronic problem.3
Signs that gout is progressing3
- More frequent and longer-lasting flare-ups
- Flare-ups in parts of the body other than the big toe (such as the knee and ankle)
- Formation of “tophi” under the skin: Tophi are uric acid crystals that get deposited under the skin and form nodules; these nodules can appear anywhere on the body and can become disfiguring without medical attention
- Kidney problems, including gouty kidney, kidney stones, and kidney failure
What can I do to help prevent gout flares?
- Drink more water4
- Limit or eliminate intake of beer, wine or spirits5
- Avoid fructose beverages, such as sugary sodas6
- Avoid foods high in purines, such as shellfish and meats, which can raise uric acid levels7
- Avoid stress, which can make gout worse8
- Learn about medications that may be right for you8; always check with your doctor before starting any new medication or diet plan.
Colchicine: Evidence-based efficacy
, the active ingredient in Mitigare®, can help prevent gout flares in adults.9
Fewer total flares with colchicine 0.6 mg than with placebo9
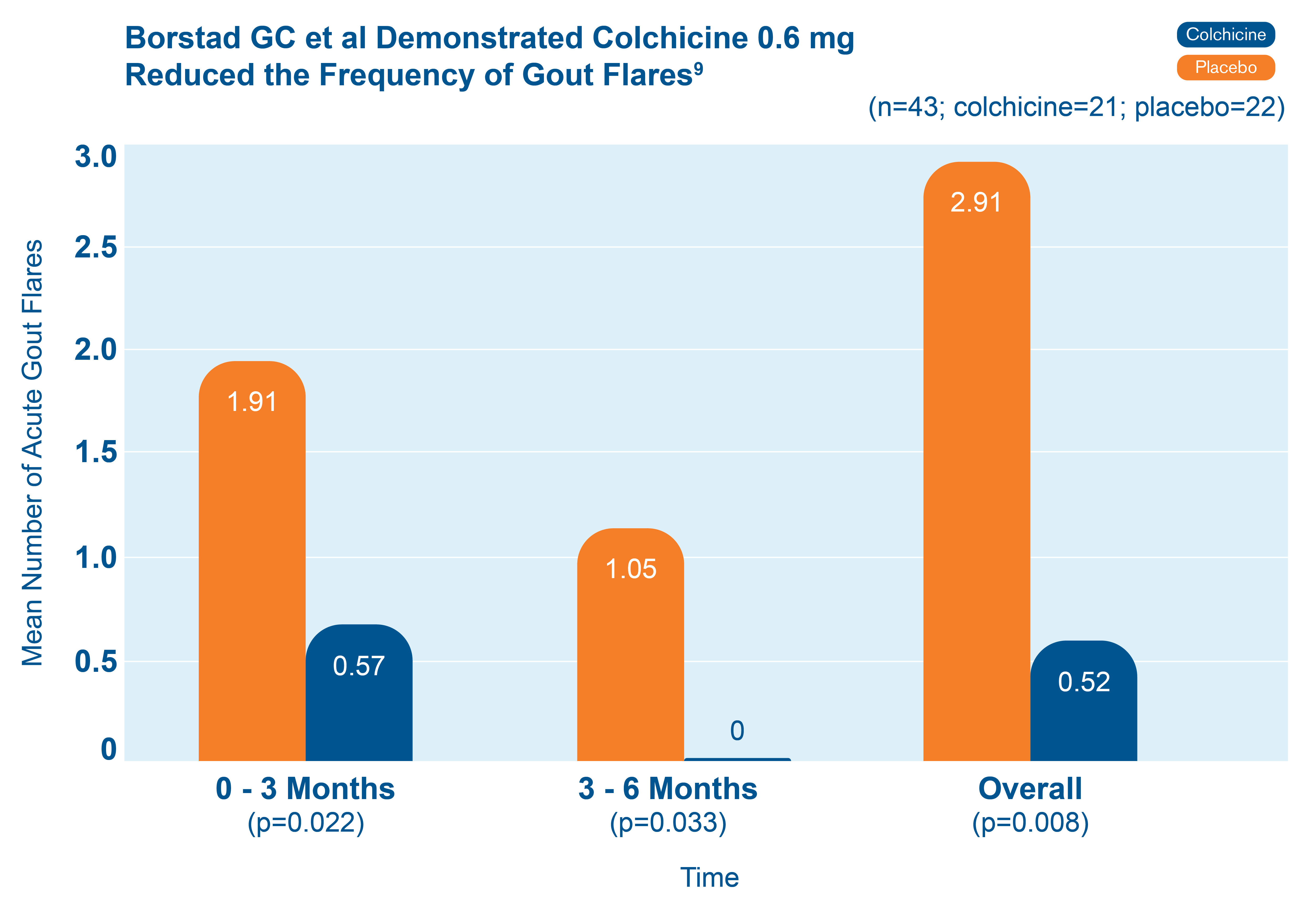
43 patients starting allopurinol were randomized to receive colchicine 0.6 mg or placebo for up to 6 months. The chart shows the mean number of acute gout flares at the 0-3 and 3-6 month time periods, and overall.9
An established safety profile
In a six-month study of prophylactic use in adult patients with recurrent gout, colchicine 0.6 mg was shown to be well tolerated.9 The most commonly reported adverse reactions with colchicine are gastrointestinal symptoms, including diarrhea, nausea, vomiting, and abdominal pain. Consult with your doctor about other medications you are currently prescribed.
The safety and effectiveness of Mitigare® for acute treatment of gout flares during prophylaxis has not been studied. Mitigare® is not an analgesic medication and should not be used to treat pain from other causes.
Important Safety Information
Consult with your doctor about other medications you are currently prescribed. Colchicine 0.6 mg capsules are contraindicated in patients with renal or hepatic impairment who are currently prescribed drugs that inhibit both P-gp and CYP3A4. Combining these dual inhibitors with colchicine in patients with renal or hepatic impairment has resulted in life-threatening or fatal colchicine toxicity. Patients with both renal and hepatic impairment should not be given Mitigare®.
Fatal overdoses have been reported with colchicine in adults and children. Keep Mitigare® out of the reach of children. Blood dyscrasias such as myelosuppression, leukopenia, granulocytopenia, thrombocytopenia, and aplastic anemia have been reported with colchicine used in therapeutic doses. Monitor for toxicity and if present consider temporary interruption or discontinuation of colchicine.
NOTE: This article was not written by a medical professional and is not intended to substitute the guidance of a physician. These are not West-Ward’s recommendations for gout flare prevention, but rather facts and data collected from various reliable medical sources. For a full list of resources and their attributing links, see below.
