
Any time you start on a new medication, it is important to consider how it might interact with the other medicines, vitamins or supplements you are already taking. What are some medications that may interact with Mitigare®? Read on to learn more.
Understanding gout
Gout is a painful form of inflammatory arthritis.1 Approximately 8.3 million Americans live with the disease.1 It usually appears first in the big toe, but can also affect the midfoot, ankles, knees, elbows, wrists and hands.2 In addition to swelling, gout can cause tenderness, redness and/or warmth in the affected joint(s), as well as lingering discomfort and limited range of motion.2
How Mitigare® helps prevent gout flares in adults
is the active ingredient in Mitigare®.3 It was originally derived from a plant called the autumn crocus (Colchicum autumnale), and there is evidence that it was used more than 2000 years ago in ancient Greece.4
Since then, colchicine has been proven effective in helping prevent flares in adults with gout.5 In one study, adults who took colchicine 0.6 mg daily had fewer gout flares than patients who did not.5 Adults who took colchicine also had fewer gout flares as time went on.5
Colchicine 0.6 mg reduced the frequency of gout flares5
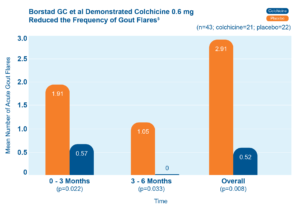
43 patients starting allopurinol were randomized to receive colchicine 0.6 mg or placebo for up to 6 months.
The chart shows the mean number of acute gout flares at the 0–3 and 3–6 month time periods and overall.5
The safety and effectiveness of Mitigare® for acute treatment of gout flares during prophylaxis has not been studied. Mitigare® is not an analgesic medication and should not be used to treat pain from other causes.3
Medications that may interact with Mitigare®
Before you take Mitigare®, tell your healthcare provider about all of your medications, including prescriptions, over-the-counter medicines, vitamins and/or herbal supplements. Using Mitigare® with certain other medicines can affect each other causing serious side effects and/or death. Do not take Mitigare® with other medicines unless your healthcare provider tells you to. It is especially important to tell your healthcare provider if you take:
- Medicines that affect how your liver works (ie, CYP3A4 inhibitors)
- A cyclosporine (eg, Neoral®, Gengraf®, Sandimmune®)
- Cholesterol-lowering medication
- Antibiotics
If you are not sure if you take any of the medicines noted above, be sure to ask your healthcare provider or pharmacist. This is not a complete list of all the medicines that can affect Mitigare®. There is additional safety information you should know about Mitigare®. Patients with both renal and hepatic impairment should not be given colchicine. The most commonly reported adverse reactions with colchicine are gastrointestinal symptoms, including diarrhea, nausea, vomiting and abdominal pain.3 If your doctor prescribes colchicine, make sure he or she knows about any other medications you are currently taking. For complete product information on Mitigare®, please see the package insert including the Medication Guide for patients.
Consider keeping a list of your medications with you to show your doctor and/or pharmacist each time you are prescribed a new medicine.
Taking your Mitigare®
Take Mitigare® exactly how your healthcare provider tells you to take it. Never start or stop a medication without first consulting with your doctor. If you have questions about Mitigare® or any of your other medications, be sure to ask your healthcare provider.
All trademarks and registered trademarks mentioned in this article are the property of their respective owners.
NOTE: This article was not written by a medical professional and is not intended to substitute for the guidance of a physician. These are not Hikma’s recommendations for gout flare prevention, but rather facts and data collected from various reliable medical sources. For a full list of resources and their attributing links, see below.
