
Gout is a type of arthritis caused by a build-up of uric acid crystals in the joints and other areas of the body and surrounding tissues. These crystals come from the body’s breakdown of purines, which occur naturally in your tissues as well as in the food you eat. Adults with gout suffer from episodes of pain, redness, swelling and/or extreme tenderness in a joint, most commonly the big toe.1 Left untreated, the risk of a second gout attack after a year is 62 percent. The risk of a second attack increases to 78 percent after 2 years, and 93 percent after 10 years.2
Fortunately for adults with gout, medications to help prevent gout flares are available; one such medication that your doctor may prescribe is colchicine. Colchicine, which is derived from a plant known as the autumn crocus or meadow saffron, was discovered centuries ago. Colchicine is now FDA-approved to help prevent gout flares in adults.3
Efficacy proven by evidence
, the active ingredient in Mitigare®, has been proven effective in helping prevent flares in adults with gout.3
• Patients who took colchicine 0.6 mg daily had fewer gout flares than patients who did not.3 Patients who took colchicine also had fewer gout flares as time went on.3
Fewer total flares with colchicine 0.6 mg than with placebo3
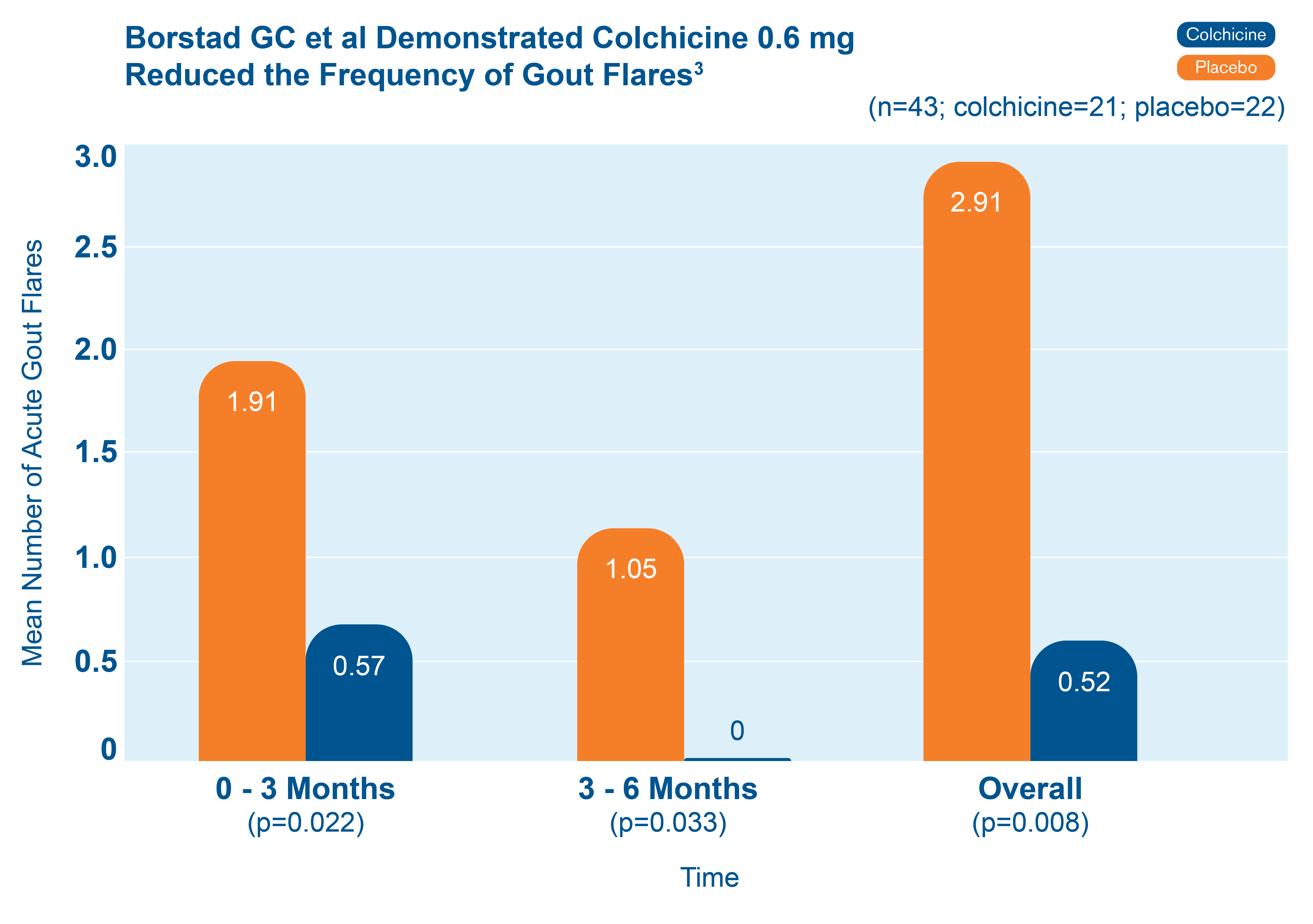
43 patients starting allopurinol were randomized to receive colchicine 0.6 mg or placebo for up to 6 months. The chart shows the mean number of acute gout flares at the 0-3 and 3-6 month time periods, and overall.3
Preventive colchicine use is recommended by the American College of Rheumatology (ACR) Guidelines
The ACR Task Force Panel (TFP) recommends oral colchicine as a first-line option for the prevention of gout-flare attacks where urate-lowering therapy (ULT) is already initiated. The ACR TFP also recommends as a first-line option (with a lower evidence grade than for colchicine) the use of low-dose non-steroidal anti-inflammatory drugs.4
Colchicine is affordable for most patients
In 2009, the FDA approved the first branded version of colchicine. Some time after issuing that approval, the FDA ordered the removal of all other colchicine products from the market because they had not yet gone through the FDA’s review process. Colchicine patients who tried to refill their prescriptions found that their previous colchicine medications were no longer available.
The only prescription option was the newly-approved branded drug offered by a pharmaceutical company that charged $5 per pill—50 times more than the price of previously available colchicine pills.5
“With the steep price increase in colchicine, many patients with gout were now unable to afford chronic colchicine therapy,” Dr. E. William St. Clair, a rheumatologist at Duke University School of Medicine, said in an interview.5
Eventually, other, more reasonably priced colchicine products received FDA approval and became available for the prevention of gout flares in adults. One such colchicine product is called Mitigare® (colchicine) 0.6 mg Capsules, now available through West-Ward Pharmaceuticals Corp.6
West-Ward Pharmaceuticals Corp. introduced its authorized generic, colchicine 0.6 mg capsules, immediately following the FDA’s approval of the branded product, Mitigare® (colchicine) 0.6 mg Capsules. West-Ward’s colchicine 0.6 mg capsule is the only colchicine therapy available in capsule form and the first authorized generic, which could offer significant potential savings to patients.
Color matters
For older adults who take more than one medicine, they should be able to tell them apart by size, shape, color and form (tablet or capsule). FDA has said that medication color can be useful in preventing medication errors.7
It is important to note that the Mitigare® (colchicine) 0.6 mg Capsule, and its authorized generic, colchicine 0.6 mg capsule, come in a distinctive, two-tone blue capsule. The unique color may help you distinguish this drug from other medications you take.8
Important Safety Information
The safety and effectiveness of Mitigare® for acute treatment of gout flares during prophylaxis has not been studied. Mitigare® is not an analgesic medication and should not be used to treat pain from other causes. Consult with your doctor about other medications you are currently prescribed. Colchicine 0.6 mg capsules are contraindicated in patients with renal or hepatic impairment who are currently prescribed drugs that inhibit both P-gp and CYP3A4. Combining these dual inhibitors with colchicine in patients with renal or hepatic impairment has resulted in life-threatening or fatal colchicine toxicity. Patients with both renal and hepatic impairment should not be given Mitigare®.
Fatal overdoses have been reported with colchicine in adults and children. Keep Mitigare® out of the reach of children. Blood dyscrasias such as myelosuppression, leukopenia, granulocytopenia, thrombocytopenia, and aplastic anemia have been reported with colchicine used in therapeutic doses. Monitor for toxicity and if present consider temporary interruption or discontinuation of colchicine. The most commonly reported adverse reactions with colchicine are gastrointestinal symptoms, including diarrhea, nausea, vomiting and abdominal pain.
NOTE: This article was not written by a medical professional and is not intended to substitute the guidance of a physician. These are not West-Ward’s recommendations for gout flare prevention, but rather facts and data collected from various reliable medical sources. For a list of resources and their attributing links, see below.
